When Science Fiction Becomes Science: The Future of Health Wearables (Part 3)
From devices to patches: simplicity is the ultimate sophistication.
This article serves as a follow-up to my initial piece on Health Wearables.
Imagine a future where health monitoring transcends traditional devices and embraces lightweight wireless sensors or even printable wearable technology. In this visionary world, simplicity is the name of the game, as the shift from bulky gadgets to sleek patches represents the pinnacle of refined innovation.
Here's an intriguing thought: perhaps we won't have to wait a whole decade or two to experience a simpler and more sophisticated approach to monitoring our health.
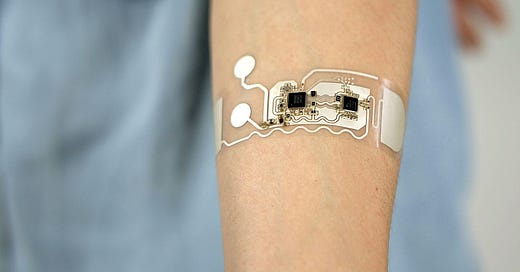
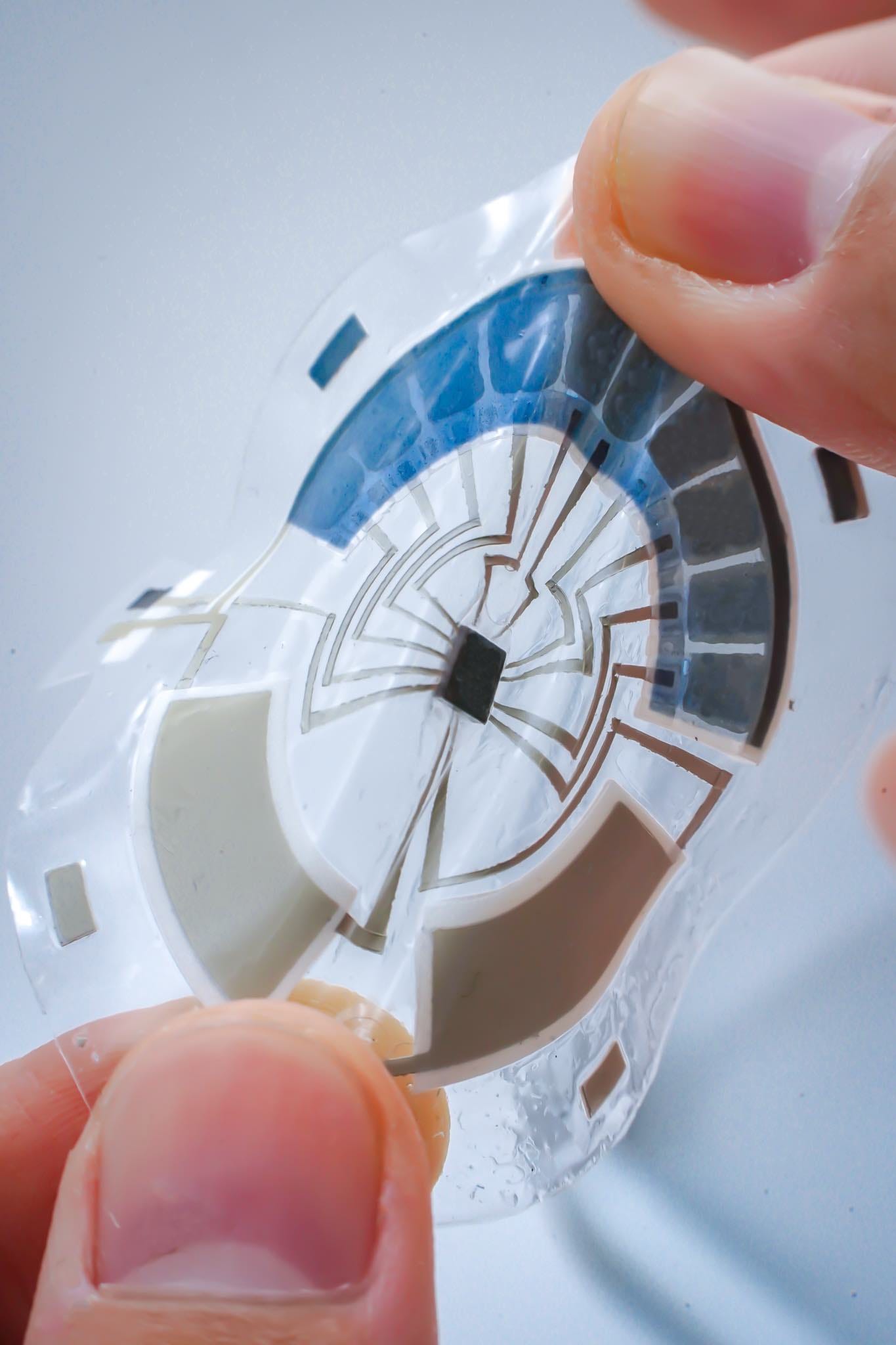
In line with this visionary future, the recent breakthrough at the University of California San Diego introduces a standalone sweat sensor that revolutionizes health monitoring. This thin, flexible, and touch-sensitive device displays various sweat parameters such as glucose, lactate, sodium, or pH levels without the need for any wired or wireless connections to external gadgets, bringing us closer to a simpler and more sophisticated approach to tracking our well-being.
This small disk-shaped patch boasts a unique design, incorporating all the vital components necessary for a wearable sensor. It features two integrated batteries, a microcontroller, sensors, a circuit, and even a stretchable display. With this comprehensive setup, the patch covers all the essential functionalities required for operating a wearable sensor, from powering up to presenting the user with clear and concise results. Who would have thought that such a compact device could pack such power and convenience? The future of health monitoring has truly arrived.
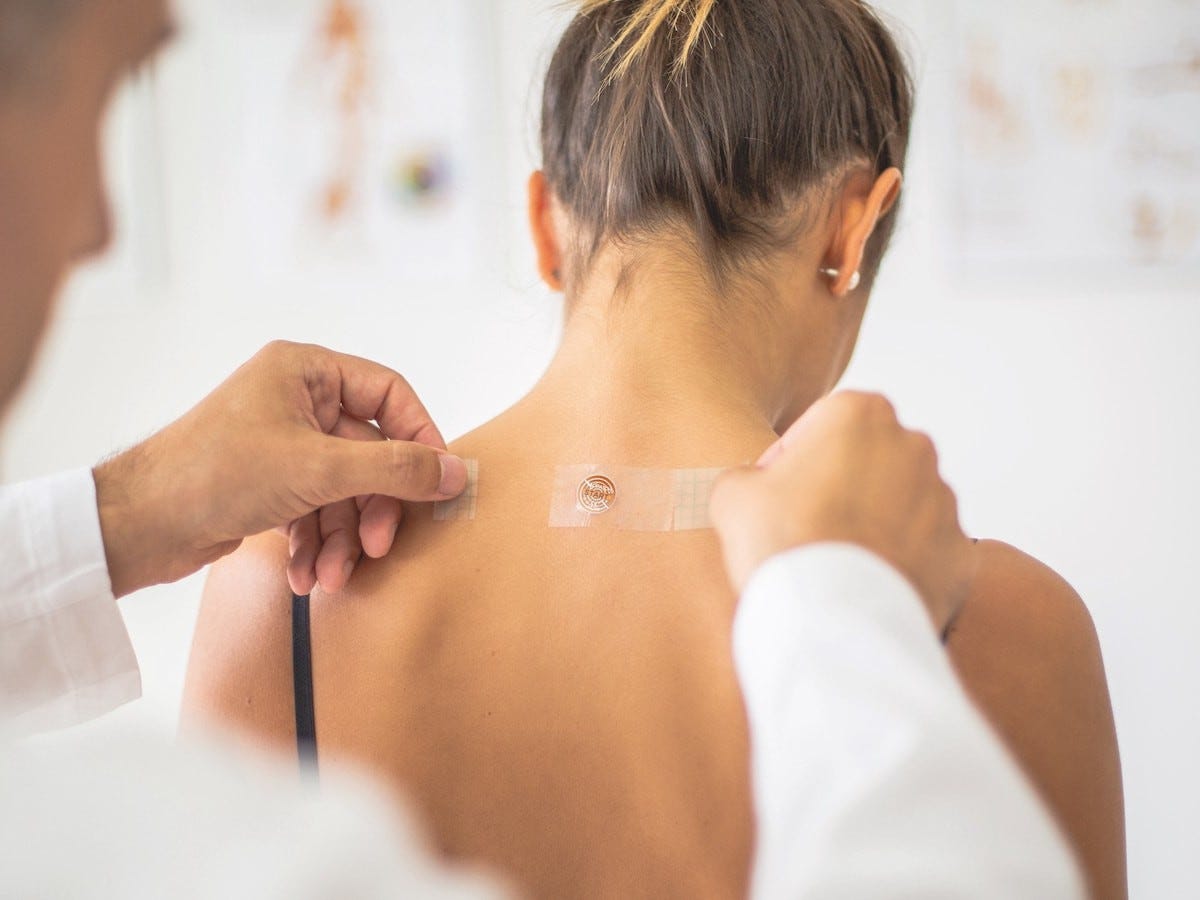
TaoPatch, Djockovic’s Biggest Secret is a patented wearable nanotechnology device that combines acupuncture with light therapy. It contains layers of nanocrystals, which capture body’s heat and convert it into impulses of very weak light, which is then emitted onto the specific points on the body. This light stimulates the Central Nervous System and enhances its communication with the rest of the human body.
The main advantages? Taopatch helps the human body rebalance itself, which leads to a surprising number of benefits: from improving posture, balance and flexibility, to boosting athletic performance and focus, and reducing stress, anxiety and chronic pain.
The emergence of advanced wearable sensors represents a significant leap forward in health monitoring. As technology continues to evolve, it is essential to consider the regulatory landscape surrounding the industry. The development of innovative and compact devices like this raises questions about how regulatory bodies in the EU and the USA will adapt to accommodate such advancements.
In both regions, health wearables are subject to regulations and face certain barriers:
In the European Union, the primary regulation governing health wearables is the General Data Protection Regulation (GDPR). The GDPR establishes rules for the processing and protection of personal data, including health-related data collected by wearables. It requires companies to obtain explicit consent from individuals before collecting and using their personal data, including health information. The GDPR also grants individuals the right to access their personal data, request its deletion, and be informed about how their data is being processed.
Additionally, in the EU, health wearables that are considered medical devices are subject to the requirements of the Medical Devices Regulation (MDR) or the In Vitro Diagnostic Medical Devices Regulation (IVDR), depending on the specific classification of the device. These regulations set out the standards and procedures for placing medical devices on the market, including safety and performance requirements, conformity assessments, and post-market surveillance.
In the United States, health wearables are regulated by various laws and regulations. One important regulation is the Health Insurance Portability and Accountability Act (HIPAA). HIPAA establishes rules for the protection and privacy of individuals' health information held by covered entities, such as healthcare providers, health plans, and healthcare clearinghouses. While HIPAA primarily applies to these covered entities, certain health wearables may fall under its scope if they are used by covered entities or if the wearable manufacturers have a business relationship with covered entities.
Furthermore, the U.S. Food and Drug Administration (FDA) regulates health wearables that qualify as medical devices. The FDA categorizes medical devices into different classes based on their level of risk. Class I devices, such as basic fitness trackers, are subject to general controls, while higher-risk Class II and Class III devices require premarket clearance or approval from the FDA. The FDA also provides guidance on software as a medical device (SaMD) and mobile medical applications (apps) to help determine regulatory requirements for health-related software used in wearables.
Currently, tech companies can avoid regulations by not categorizing smartwatches as healthcare devices. This allows them to bypass certain requirements, such as obtaining explicit consent from individuals to share sensitive health information, as mandated by HIPAA. However, as smartwatches become integrated into electronic health records (EHRs) and their alerts lead more patients to seek healthcare services, regulators may enforce stricter rules upon these companies.
A critical challenge for the industry to sustain its growth lies in finding the right balance between fostering innovation and ensuring data privacy, safety, and accuracy. This balance will be pivotal as wearable sensor technology continues to shape the future landscape of health monitoring. It is precisely this balance that defines the essence of innovation, isn't it?
+ endurance is good,
Martin
P.S. I used some of the following sources to obtain the data for this article:
(1) UCSD Center for Wearable Sensors, (2) European Commission , (3) FDA, (4) HIPAA